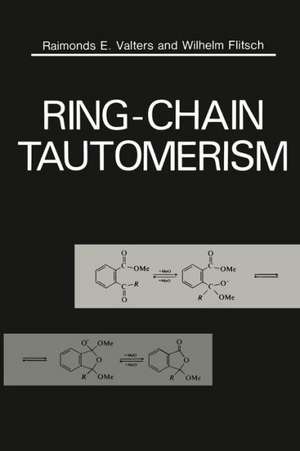
Ring-Chain Tautomerism
Autor Raimonds Valtersen Limba Engleză Paperback – 14 mar 2012
Preț: 387.38 lei
Nou
Puncte Express: 581
Preț estimativ în valută:
74.13€ • 76.93$ • 61.96£
74.13€ • 76.93$ • 61.96£
Carte tipărită la comandă
Livrare economică 15-29 martie
Preluare comenzi: 021 569.72.76
Specificații
ISBN-13: 9781468448856
ISBN-10: 1468448854
Pagini: 296
Ilustrații: XII, 278 p.
Dimensiuni: 152 x 229 x 16 mm
Greutate: 0.4 kg
Ediția:Softcover reprint of the original 1st ed. 1985
Editura: Springer Us
Colecția Springer
Locul publicării:New York, NY, United States
ISBN-10: 1468448854
Pagini: 296
Ilustrații: XII, 278 p.
Dimensiuni: 152 x 229 x 16 mm
Greutate: 0.4 kg
Ediția:Softcover reprint of the original 1st ed. 1985
Editura: Springer Us
Colecția Springer
Locul publicării:New York, NY, United States
Public țintă
ResearchCuprins
1. Introduction.- 1.1. Ring-Chain Isomeric Interconversions. General Considerations.- 1.2. Tautomerism or Isomerism? System Mobility and Equilibrium Position.- 1.3. Methods of Investigation of Ring-Chain Addition Tautomerism.- References.- 2. Intramolecular Reversible Addition Reactions to the C=O Group.- 2.1. Aldehydo and Keto-carboxylic Acids and Their Derivatives Modified at the Carboxylic Group.- 2.2. Hydroxy Aldehydes and Ketones and Related Compounds.- 2.3. Amino Aldehydes and Ketones and Related Compounds.- 2.4. Intramolecular Migration of O-, N-, and S-Acyl Groups.- 2.5. Oxa-, Aza-, and Thiacyclols.- 2.6. Mercapto Aldehydes and Ketones and Related Compounds.- 2.7. Intramolecular Addition of C-H Groups.- References.- 3. Intramolecular Reversible Addition Reactions to the C=N Group.- 3.1. OH-Derivatives of Imines, Hydrazones, Oximes, and Nitrones.- 3.2. N-H-Derivatives of Imines, Hydrazones, Oximes, and Nitrones.- 3.3. S-H-Derivatives of Imines and Hydrazones.- 3.4. Miscellaneous.- References.- 4. Intramolecular Reversible Addition Reactions to Other Groups.- 4.1. Addition to the C?N Group.- 4.2. Addition to C=C and C?C Groups.- 4.3. Addition to the P=O Group.- 4.4. Addition to the P=N Group.- 4.5. Addition to the S=O Group.- 4.6. Addition to the Se=O Group.- 4.7. Addition to the I=O Group.- References.- 5. Generalizations Concerning the Influence of Structural and External Factors on the Relative Stability of Ring and Chain Isomers.- 5.1. Structural Influences.- 5.2. Influence of External Factors.- References.