Pharmaceutical Salts and Co-Crystals: RSC Drug Discovery, cartea 16
en Limba Engleză Hardback – 13 noi 2011
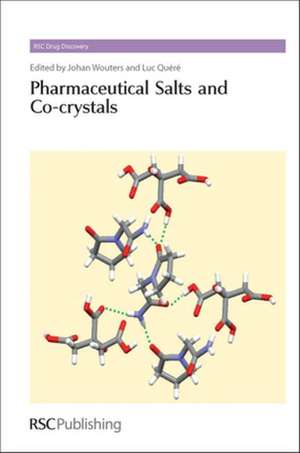
From crystal structure prediction to totally empirical screening, the quest for new crystal forms has become one of the most challenging issues in the solid state science and particularly in the pharmaceutical world. In this context, multi-component crystalline materials like co-crystals have received renewed interest as they offer the prospect of optimized physical properties. As illustrated in this first book_ entirely dedicated to this emerging class of pharmaceutical compounds_ the outcome of such endeavours into crystal engineering have demonstrated clear impacts on production, marketing and intellectual property protection of active pharmaceutical ingredients (APIs). Indeed, co-crystallization influences relevant physico-chemical parameters (such as solubility, dissolution rate, chemical stability, melting point, hygroscopicity, …) and often offers solids with properties superior to those of the free drug. Combining both reports of the latest research and comprehensive overviews of basic principles, with contributions from selected experts in both academia and industry, this unique book is an essential reference, ideal for pharmaceutical development scientists and graduate students in pharmaceutical science.
Din seria RSC Drug Discovery
- 14%
 Preț: 1227.21 lei
Preț: 1227.21 lei - 27%
 Preț: 2320.97 lei
Preț: 2320.97 lei - 5%
 Preț: 1548.26 lei
Preț: 1548.26 lei - 5%
 Preț: 1146.35 lei
Preț: 1146.35 lei - 5%
 Preț: 1137.19 lei
Preț: 1137.19 lei - 14%
 Preț: 1028.99 lei
Preț: 1028.99 lei - 5%
 Preț: 1139.05 lei
Preț: 1139.05 lei - 14%
 Preț: 1080.91 lei
Preț: 1080.91 lei - 14%
 Preț: 1195.25 lei
Preț: 1195.25 lei - 14%
 Preț: 1137.86 lei
Preț: 1137.86 lei - 5%
 Preț: 1372.96 lei
Preț: 1372.96 lei - 14%
 Preț: 1247.24 lei
Preț: 1247.24 lei - 9%
 Preț: 1067.06 lei
Preț: 1067.06 lei - 14%
 Preț: 1040.85 lei
Preț: 1040.85 lei - 14%
 Preț: 1163.73 lei
Preț: 1163.73 lei - 14%
 Preț: 1247.53 lei
Preț: 1247.53 lei - 5%
 Preț: 1554.98 lei
Preț: 1554.98 lei - 14%
 Preț: 1359.11 lei
Preț: 1359.11 lei - 5%
 Preț: 1529.80 lei
Preț: 1529.80 lei - 14%
 Preț: 1381.56 lei
Preț: 1381.56 lei - 5%
 Preț: 1440.92 lei
Preț: 1440.92 lei - 5%
 Preț: 1138.48 lei
Preț: 1138.48 lei - 5%
 Preț: 1535.29 lei
Preț: 1535.29 lei - 14%
 Preț: 1383.21 lei
Preț: 1383.21 lei - 14%
 Preț: 1389.84 lei
Preț: 1389.84 lei - 14%
 Preț: 1321.37 lei
Preț: 1321.37 lei - 5%
 Preț: 1497.68 lei
Preț: 1497.68 lei - 14%
 Preț: 1033.62 lei
Preț: 1033.62 lei - 14%
 Preț: 1134.26 lei
Preț: 1134.26 lei - 5%
 Preț: 1240.14 lei
Preț: 1240.14 lei - 5%
 Preț: 1239.41 lei
Preț: 1239.41 lei - 14%
 Preț: 947.56 lei
Preț: 947.56 lei - 14%
 Preț: 777.54 lei
Preț: 777.54 lei - 14%
 Preț: 1136.40 lei
Preț: 1136.40 lei - 14%
 Preț: 1241.40 lei
Preț: 1241.40 lei - 5%
 Preț: 1499.54 lei
Preț: 1499.54 lei - 5%
 Preț: 886.60 lei
Preț: 886.60 lei - 5%
 Preț: 964.67 lei
Preț: 964.67 lei - 5%
 Preț: 1052.39 lei
Preț: 1052.39 lei - 9%
 Preț: 1068.32 lei
Preț: 1068.32 lei - 9%
 Preț: 764.05 lei
Preț: 764.05 lei - 9%
 Preț: 1110.77 lei
Preț: 1110.77 lei - 5%
 Preț: 1160.16 lei
Preț: 1160.16 lei - 5%
 Preț: 1157.56 lei
Preț: 1157.56 lei - 5%
 Preț: 1158.06 lei
Preț: 1158.06 lei - 5%
 Preț: 1157.11 lei
Preț: 1157.11 lei - 9%
 Preț: 1211.71 lei
Preț: 1211.71 lei - 5%
 Preț: 1120.12 lei
Preț: 1120.12 lei
Preț: 1006.30 lei
Preț vechi: 1105.83 lei
-9% Nou
Puncte Express: 1509
Preț estimativ în valută:
192.60€ • 200.29$ • 161.16£
192.60€ • 200.29$ • 161.16£
Carte indisponibilă temporar
Doresc să fiu notificat când acest titlu va fi disponibil:
Se trimite...
Preluare comenzi: 021 569.72.76
Specificații
ISBN-13: 9781849731584
ISBN-10: 1849731586
Pagini: 406
Ilustrații: 152 colour illustrations
Dimensiuni: 163 x 241 x 27 mm
Greutate: 0.75 kg
Editura: Royal Society Of Chemistry
Seria RSC Drug Discovery
ISBN-10: 1849731586
Pagini: 406
Ilustrații: 152 colour illustrations
Dimensiuni: 163 x 241 x 27 mm
Greutate: 0.75 kg
Editura: Royal Society Of Chemistry
Seria RSC Drug Discovery
Cuprins
Fundamental Aspects on Salts and Co-crystals; Preparative Methods for Co-crystals; Characterization of Co-crystals and Salts; Applications of Co-crystals and Salts in Pharmaceutical Solid State Property Management
Notă biografică
Johan Wouters is Professor at the Department of Chemistry and a Member of the Drug Design Discovery Center at the University of Namur, Belgium. He is also a teacher in general chemistry, crystallography and structure-based drug design and is the author of about 120 papers. Luc Quéré is at UCB, a global Biopharma, as head of Chemical Analytical Development. Current UCB strategy with respect to solid-state issues and opportunities has been developed under his leadership.
Textul de pe ultima copertă
Multi-component crystalline materials (salts and co-crystals) have received renewed interest in recent years due to their importance in the pharmaceutical industry. Pharmaceutical salts and co-crystals represent an emerging class of pharmaceutical materials offering the prospect of optimized physical properties giving new stable and patentable solid forms. Indeed these multi-component crystalline forms influence relevant physico-chemical parameters such as solubility, dissolution rate, chemical and physical stability, melting point and hygroscopicity, which can result in solids with properties superior to those of the free drug. This unique book focuses on the currently 'hot topic' of Pharmaceutical Salts and Co-crystals and covers the following key topics: the fundamental aspects of salts and co-crystals; the role of fluorine in weak interactions in co-crystals; polymorph prediction of small organic molecules, co-crystals and salts; shape and polarity in co-crystal formation; the role of co-crystals in pharmaceutical development; solid forms and pharmacokinetics; the application of mechanochemistry in the synthesis and discovery of new pharmaceutical forms; co-crystallization in solution and scale-up; analytical techniques and strategies for salt/co-crystal characterization; co-crystal solubility and thermodynamic stability; application of phase diagrams in co-crystal search and preparation; limits of the co-crystal concept and beyond; commercial opportunities and patent considerations; using Piracetam as a learning model and monographs of the most frequent co-crystal formers. Combining both reports of the latest academic research and comprehensive overviews of basic principles, with more applied contributions from selected experts in industry. This blend of expertise makes this book an essential reference for pharmaceutical development scientists, researchers in crystal engineering, and is equally useful for the more seasoned expert as well as for the graduate student.
Descriere
This book covers the hot topic of pharmaceutical salts and co-crystals focusing on the following essential aspects: an overview of fundamental aspects on salts and co-crystals, racemic resolution via diastereomer separation, optimization of relevant physico-chemical parameters, and strengthening of intellectual property.